
In order to implement the spirit of the relevant documents of the Ministry of Industry and Information Technology, the National Health Commission and the National Food and Drug Administration, seize the new opportunities for the development of biological economy and digital economy, and further promote the development of intelligent and digital medical devices, The “2023 Intelligent Medical Device Innovation Conference and Suzhou High-end Medical Device Industry Innovation and Development Forum” sponsored by Suzhou Municipal People’s Government and China Academy of Information and Communication Technology was held in Suzhou High-tech Zone from October 26 to 27, 2023.
At the meeting, the list of finalists for the artificial intelligence medical device innovation task of the Ministry of Industry and Information Technology in Suzhou was grand released. Big Vision Medical became one of the only eight finalists in Suzhou with its advanced ophthalmic OCT image auxiliary diagnosis software (MIAS-3000).
The conference focused on seven sub-fields of artificial intelligence medical devices, telemedicine devices, medical device network security, digital therapy, and brain-computer interface. Well-known academicians and industry experts were invited to gather at the scene to discuss cutting-edge innovative technologies of intelligent medical devices, deeply interpret industry policies, and analyze industry macro trends.
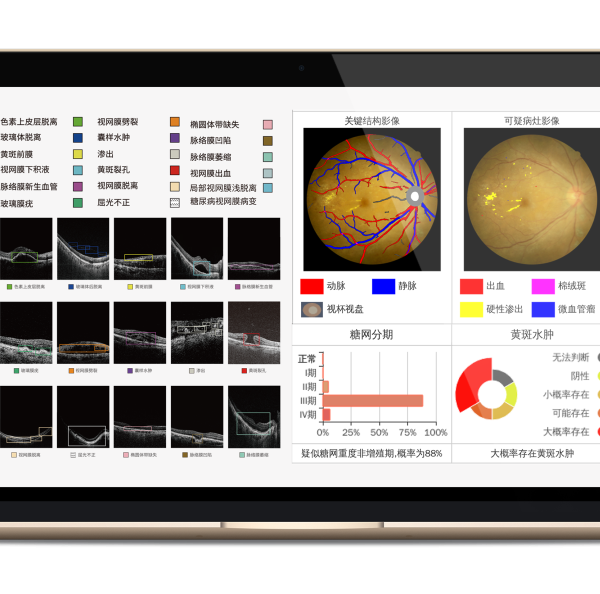
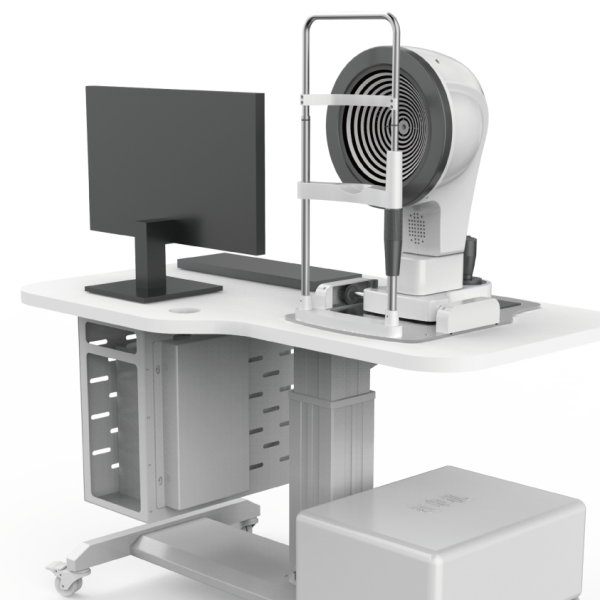
As a leader in ophthalmic AI technology, Big Vision Medical focuses on the development and innovation of ophthalmic AI software and hardware systems. The ophthalmic OCT image auxiliary diagnosis software MIAS-3000, which is one of the finalists in the Innovation task opening project, can complete the automatic auxiliary diagnosis of age-related macular degeneration (AMD) diseases in middle-aged and elderly people by using advanced AI medical imaging algorithm model. The early detection, early detection and early treatment of AMD will provide an effective solution to solve the contradiction between the huge base of eye disease patients and the shortage of medical resources in China.At present, the product has completed clinical trials, and is expected to be approved in June 2024 as the world’s first OCT image-assisted diagnosis system with Class 3 approval.

About Big Vision Medical
On November 3, 2023, Big Vision Healthcare will mark its eighth anniversary.Since the industry’s first multi-modal eye image artificial intelligence assisted diagnosis system MIAS forensics came on the market in 2018, Big Vision Medical has successively obtained medical device registration certificates for intelligent ophthalmic products such as automatic artificial intelligence OCT, vision screening instrument and AI dry eye detector developed by independent innovation. It has realized the advanced product form from a single algorithm software to a combination of soft and hard, providing users with a complete system solution, and has been widely used in ophthalmology clinic. At the same time, Big Vision Medical biometer has also entered the testing stage and is expected to be sold in June 2024.MIAS-3000 is also expected to obtain the registration certificate of three types of medical devices in the same period, leading ophthalmic AI to enter a new realm of more comprehensive and accurate.
Big Vision is deeply honored to be a finalist in the “Artificial Intelligence Medical Device Innovation Task Unveiling”, and also knows that it has a great responsibility. Looking forward to the future, Big Vision Medical has always kept in mind the original intention and mission of “early detection of every pair of eyes with problems, and the best treatment plan”. The team will adhere to independent innovation, focus on the transformation and application promotion of intelligent medical innovation, and continue to actively explore the innovative path of the integration of artificial intelligence and medical devices, and strive to promote the application of intelligent medical devices. To contribute scientific and technological strength to the realization of national eye health.














No comments yet.