Big Vision news
Recently, Jiangxi Big Vision successfully passed the ISO13485 quality system certification (its full name is “medical device quality management system for regulatory requirements”) and won the quality management system certificate, certificate number CN21/42448.

ISO 13485 is a quality management system standard applicable to the medical device regulatory environment, combining the characteristics of the medical device industry and highlighting the legal and regulatory requirements. The certificate is applicable to “the design and development, manufacture, installation and service of medical devices or the design, development and provision of related services and other related industries”.
As the mainstream framework and standard for medical device quality management, ISO13485 certification is widely recognized worldwide. the certification and operation of ISO13485 system can continuously improve and enhance the management level of enterprises, provide users with stable quality products, and help eliminate technical barriers in international trade, which is a passport to enter the international market.
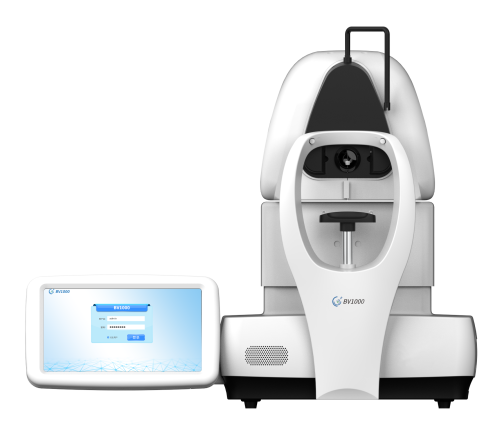
The BV1000 uses advanced algorithms to achieve automatic pupil positioning and focusing, and enables patients to complete all examinations independently through voice guidance, with a short learning curve, while integrating Big Vision’s industry-leading OCT ophthalmic imaging artificial intelligence algorithms, allowing patients to receive examination reports within 2 minutes of completion, effectively alleviating the pain of insufficient numbers of professional ophthalmologists in primary hospitals. The BV1000 examination results can also be seamlessly connected to Big Vision’s cloud-based Bright Lemon eye health management platform, which allows users to establish and maintain eye health records that can be viewed and tracked at any time, thus creating a complete eye health management ecosystem.
August 12, 2021








No comments yet.